56+ calculate the ratio of effusion rates for 235u and 238u
Question Transcribed Image Text. Web Daughter isotope determinations from the two decay systems may also be combined to calculate a 207 Pb-206 Pb date in concert with an assumed or measured present-day.
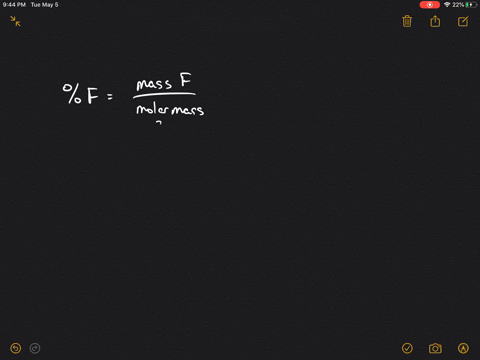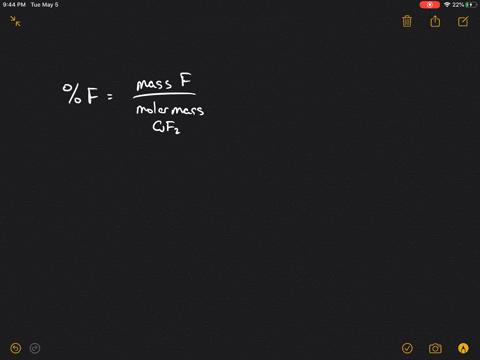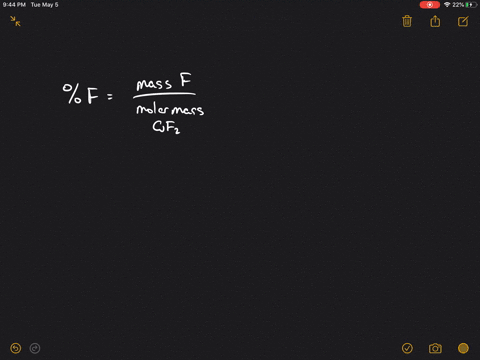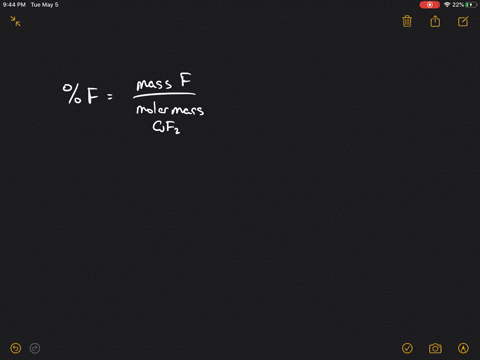
Solved A Sample Of Uranium Fluoride Is Found To Effuse At The Rate Of 17 7 Mg H Under Comparable Conditions Gaseous I2 Effuses At The Rate Of 15 0 Mg H
Molecular Effusion and Diffusion Last updated Save as PDF Page ID 21766 Learning.

. Medium View solution The rates of diffusion of two gases. Calculate the ratio of effusion rates for ar and kr. Web Calculate the ratio of effusion rates for 235u and 238u.
The atomic weights are 235 U 23504 amu. How can you use ratios and rates to solve problems. 238 U 23805 amu.
Web Calculate the ratio of rates of effusion of 235 UF 6 and 238 UF 6 where 235 U and 238 U are isotopes of uranium. What is the ratio in the form of a decimal of the RMS. 993 are 238u atomic mass 238 u.
Web The ratio of rates of diffusion of gases X and Y is 15 and that of Y and Z is 16. The ratio of rate of diffusion of Z and X is. 1086 As discussed in the.
Rate 235 U F 6 rate 238 U F 6 35204 g m o l 34903 g m o l. We have step-by-step solutions. The atomic weights are 235U 23504 amu.
Web Calculate the ratio of effusion rates for 235u and 238uand compare it to the ratio for uf6 given in the essay. Web The ratio of the effusion rates can be calculated from Grahams law using Equation 1081. Web Calculate the ratio of rates of effusion of 235UF6 and 238UF6 where 235U and 238U are isotopes of uranium.
Web The two isotopes of uranium 238U and 235U can be separated by effusion of the corresponding U F 6 gases. Web Calculate the ratio of effusion rates for 235U and 238U and compare it to the ratio for UF6 given in the essay. Gammon Chapter 5 Problem 5125QP.
Web Textbook solution for General Chemistry - Standalone book MindTap Course 11th Edition Steven D.
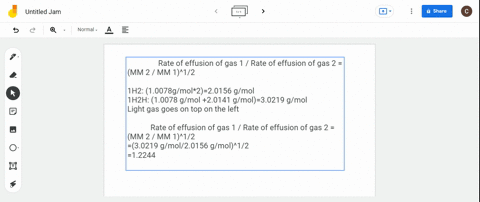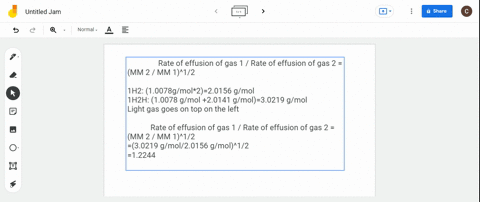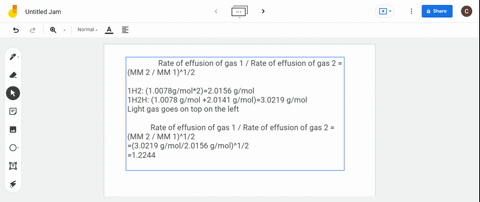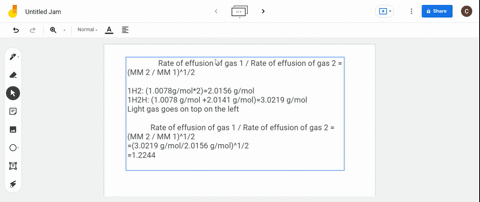
Solved Calculate The Ratio Of Rates Of Effusion Of 235 Uf6 And 238 Uf6 Where 235 U And 238 U Are Isotopes Of Uranium The Atomic Masses Are 235 U 235 04 Amu

Calculate The Relative Rates Of Diffusion Of 235uf6 And 238uf6 In Gaseous Form F 19

The Kinetic Molecular Theory Of Gases And Effusion And Diffusion Ppt Video Online Download
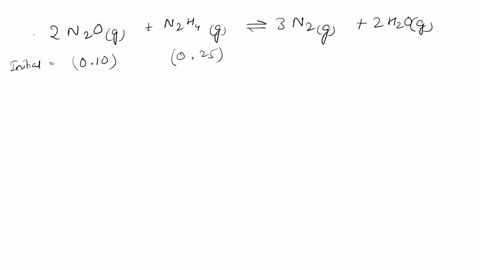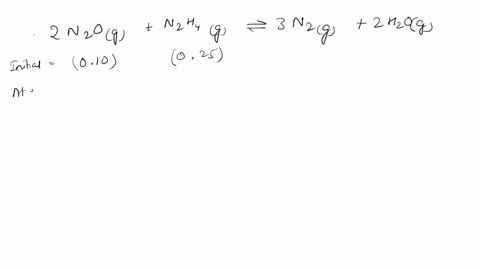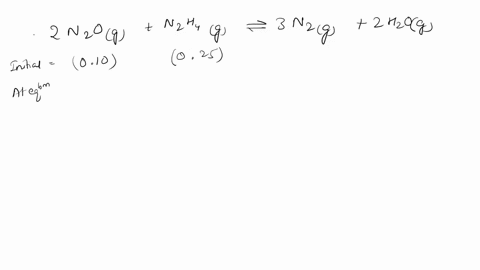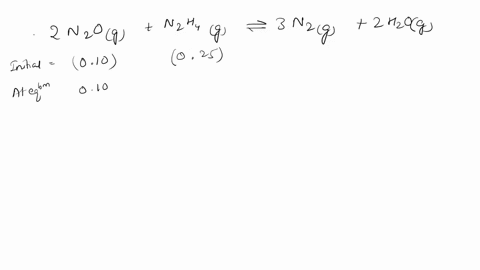
Solved Calculate The Ratio Of Rates Of Effusion Of 235 Uf6 And 238 Uf6 Where 235 U And 238 U Are Isotopes Of Uranium The Atomic Weights Are 235 U 235 04 Amu
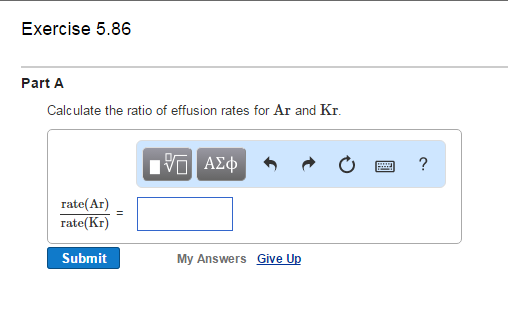
Solved Calculate The Ratio Of Effusion Rates For Ar And Kr Chegg Com

Chapter 5 Gases And The Kinetic Molecular Theory 5 1 An Overview Of The Physical States Of Matter 5 2 Gas Pressure And It S Measurement 5 3 The Gas Ppt Download

Solved A Sample Of Uranium Fluoride Is Found To Effuse At The Rate Of 17 7 Mg H Under Comparable Conditions Gaseous I2 Effuses At The Rate Of 15 0 Mg H

We Separate U 235 From U 238 By Fluorinating A Sample Of Uranium Pearson Channels
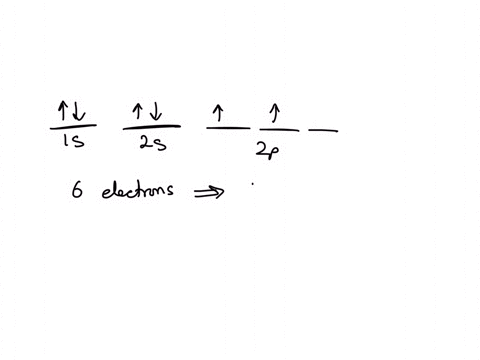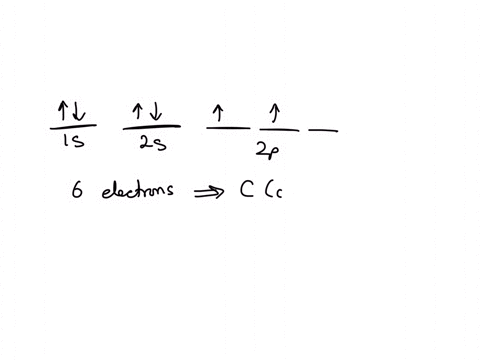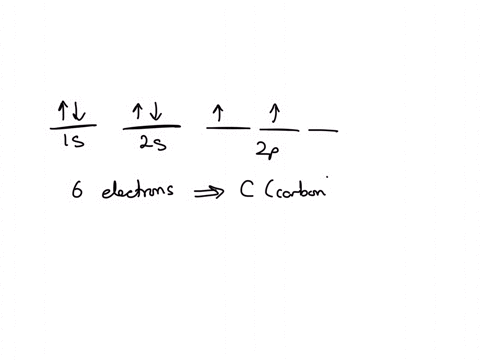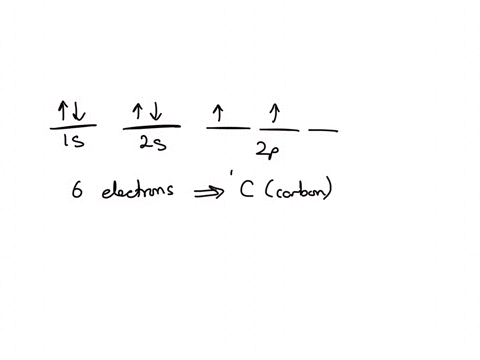
Solved Calculate The Ratio Of Rates Of Effusion Of 235 Uf6 And 238 Uf6 Where 235 U And 238 U Are Isotopes Of Uranium The Atomic Weights Are 235 U 235 04 Amu

180 Ml Of Hydrocarbon Diffuses Through A Porous Membrane In 15 Minutes While 120 Ml Of So2 Under Identical Conditions Diffuses In 20 Minutes What Is The Molecular Mass Of The Hydrocarbon

Solved Calculate The Ratio Of Rates Of Effusion Of 235 Uf6 And 238 Uf6 Where 235 U And 238 U Are Isotopes Of Uranium The Atomic Weights Are 235 U 235 04 Amu
Solved Calculate The Ratio Of Rates Of Effusion Of 235 Uf6 And 238 Uf6 Where 235 U And 238 U Are Isotopes Of Uranium The Atomic Weights Are 235 U 235 04 Amu

Calculate The Relative Rates Of Diffusion Of 235uf6 And 238uf6 In The Gaseous State At Mass Of F 19

Calculate The Relative Rates Of Diffusion For 235 Uf 6 And 238 Uf 6

Solved We Obtain Uranium 235 From U 238 By Fluorinating The Uranium To Form Uf6 Which Is A Gas And Then Taking Advantage Of The Different Rates Of Effusion And Diffusion For Compounds Containing
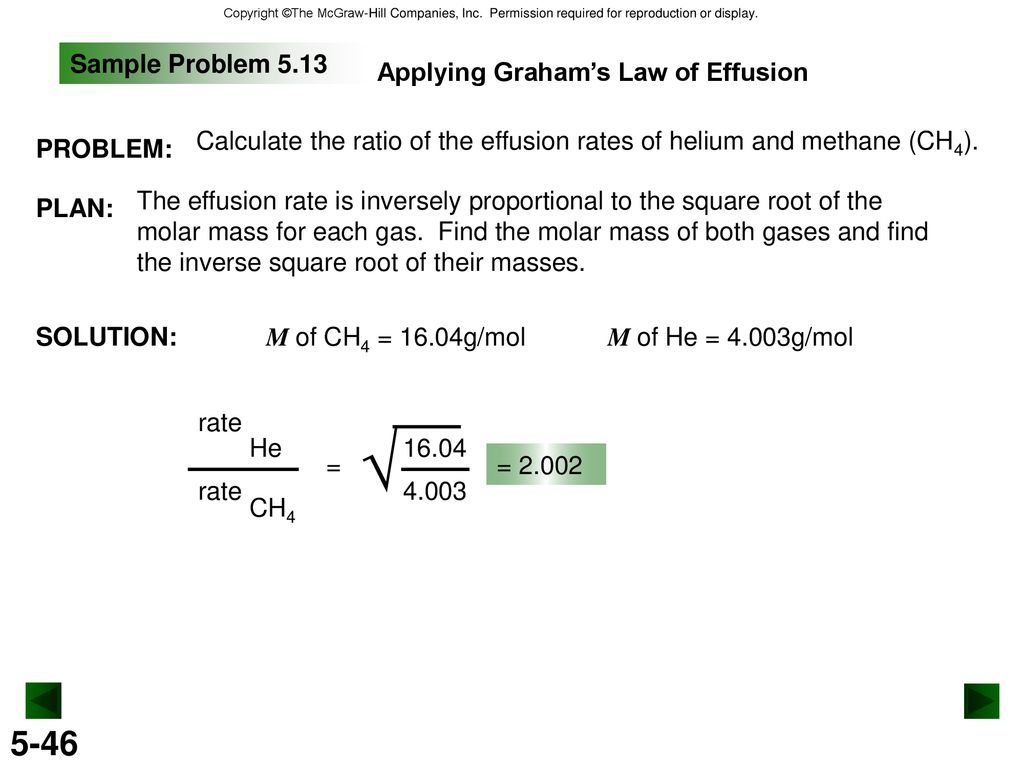
Chapter 5 Gases And The Kinetic Molecular Theory Ppt Download
Solved We Obtain Uranium 235 From U 238 By Fluorinating The Uranium To Form Uf6 Which Is A Gas And Then Taking Advantage Of The Different Rates Of Effusion And Diffusion For Compounds Containing The